Natural Grocers Wins GMO Labeling Appeal; Supplement Industry Under Pressure
This article first appeared in the February 2026 issue of Presence Marketing’s newsletter.
By Steven Hoffman
In January 2026, the regulatory framework governing the natural products industry encountered significant developments affecting how food and dietary supplements are labeled and regulated. Through a combination of judicial rulings, agency guidance, and legislative proposals, the requirements for transparency and product disclosure are shifting, presenting new compliance considerations for manufacturers and retailers alike.
For CPG brands, ingredient suppliers, and compliance officers, these updates signal a continued move toward explicit, on-package disclosure. Recent events indicate that both the courts and legislators are increasingly prioritizing clear, accessible information for consumers, challenging previous standards that allowed for digital or abbreviated disclosures.
This report outlines two primary developments from the start of the year: the U.S. Court of Appeals ruling in favor of Natural Grocers regarding Bioengineered (BE) disclosures, and a dual-front regulatory discussion involving the FDA and Senator Dick Durbin (D-IL) regarding the dietary supplement sector.
Federal Appeals Court Sides with Natural Grocers in GMO Ruling
In a decision delivered on Jan. 6, 2026, the U.S. Court of Appeals for the Ninth Circuit ruled in favor of a coalition of plaintiffs led by the Lakewood, CO-based retailer Natural Grocers by Vitamin Cottage (NYSE: NGVC) and the Center for Food Safety (CFS). The court’s decision effectively strikes down key portions of the USDA’s Bioengineered Food Disclosure Standard, addressing industry arguments that the previous rules contained exemptions that limited consumer access to information.
The "National Bioengineered Food Disclosure Standard" (NBFDS) has been a subject of debate since its inception. Critics, including the plaintiffs, argued that the USDA’s implementation allowed manufacturers to obscure the presence of genetically modified organisms (GMOs) through the use of digital links and unfamiliar terminology.
According to a Natural Grocers press release, the court’s ruling necessitates a significant revision of USDA rules. The outcome aligns with a long-standing position of Natural Grocers, the nation’s largest family-operated organic and natural grocery retailer, which has prohibited most GMO ingredients in its stores since 2012 and advocated for clearer labeling standards.
The court’s decision focused on three specific areas where the USDA’s previous rules were found to be insufficient or unlawful. Food and beverage manufacturers must now prepare for a regulatory environment that will likely require strategic adjustments in the next rulemaking cycle.
The "Bioengineered" Terminology Battle
First among the court's findings was the rejection of the USDA’s mandate that strictly required the use of the term "bioengineered." Plaintiffs successfully argued that this term is unfamiliar to the average shopper and infringed on free speech rights by prohibiting the use of terms consumers actually understand.
Under the overturned rules, a manufacturer was forced to use "bioengineered" even if their customer base was far more familiar with "GMO" or "Genetically Engineered." According to the Non-GMO Project, recent market research indicates that while 63% of consumers recognize the term "GMO," only 36% are familiar with "bioengineering." By mandating the lesser-known term, the USDA was seen as complicating disclosure. The ruling now paves the way for retailers and brands to use terms that resonate more clearly with their customers, potentially returning the familiar "GMO" acronym to federal disclosures.
Closing the Digital Divide: The End of QR Code Exclusivity
Operationally, a significant aspect of the ruling is the rejection of standalone QR codes as a sufficient means of disclosure. The USDA had previously allowed companies to forgo on-package text disclosures entirely in favor of a scannable code. Natural Grocers and the Center for Food Safety argued that this practice excluded consumers without smartphones, reliable internet access, or technical literacy—demographics that often include the elderly and rural populations.
The court agreed, ruling that companies cannot rely solely on digital disclosures. This decision impacts the "scan to learn more" approach that some large CPG companies had adopted. Brands that utilized digital links to manage label space must now redesign packaging to include clear, on-pack text or symbols accessible to the naked eye.
Highly Processed Ingredients: No More Hiding
Finally, the court found the USDA was incorrect in exempting highly processed foods—such as sugar from sugar beets or oil from canola—simply because the genetic material might not be detectable in the final refined product.
This "highly refined" exemption had been a major point of contention. Natural Grocers argued that even if the DNA is denatured or removed during processing, the ingredient still originates from a bioengineered crop system. The environmental and agricultural impacts remain, regardless of the final chemical structure of the sugar or oil.
"The court’s rejection of the ‘highly refined’ exemption reinforces an important principle: how food is made matters," noted Charlene Guzman, Communications Director of the Non-GMO Project, in a statement to Nosh. Brands that have relied on this exemption should expect closer scrutiny as the USDA revises its rules, particularly for ubiquitous ingredients like oils, sugars, and starches derived from GMO crops.
Heather Isely, Executive Vice President of Natural Grocers, stated that the decision reflects congressional intent. "Congress never intended to require the use of specific terms, the sole use of QR codes, or the exclusion of ingredients made from highly processed GMO crops," she said. "We are pleased the court recognized the shortcomings of the final rule and mandated corrections. Natural Grocers will remain actively engaged in the GMO regulatory process."
George Kimbrell, Legal Director of the Center for Food Safety, added that the ruling ensures consumers will eventually see "clear and accurate GMO label information."
The legal victory is consistent with Natural Grocers' long history of rigorous product standards. Founded in 1955 and with 168 stores across 21 states, the company has utilized a dynamic list—"Things We Won't Carry and Why"—to screen products. As stated in WholeFoods Magazine, if a company cannot verify non-GMO status, Natural Grocers will not stock the item.
The Supplement Industry’s Regulatory Tug-of-War
While the food industry assesses the implications of the GMO ruling, the dietary supplement sector is navigating a complex regulatory landscape. On one hand, the FDA is signaling potential flexibility regarding labeling requirements. On the other, Senator Dick Durbin has reintroduced legislation that could impose new registration requirements.
In a letter to the industry issued on Dec. 11, 2025, the FDA announced it is considering amendments to 21 C.F.R. § 101.93(d). This regulation currently governs the placement of the disclaimer required for structure/function claims under the Dietary Supplement Health and Education Act of 1994 (DSHEA).
Under current rules, supplements making claims such as "Supports heart health" must carry the standard disclaimer: "This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease." Regulations have historically required this disclaimer to appear on every single panel where a claim is made. For small bottles, this often leads to "label clutter," where the same disclaimer is repeated multiple times.
According to the National Law Review, the FDA is looking to remove the "each panel" requirement. Kyle Diamantas, FDA Deputy Commissioner for Human Foods, noted in the letter that revising this regulation would "reduce label clutter and unnecessary costs," aligning with the agency's historical enforcement posture.
Effective immediately, the FDA is exercising "enforcement discretion." The agency will not prioritize penalizing companies that do not repeat the disclaimer on every panel, provided the disclaimer appears at least once and is properly linked to the claims. However, companies should proceed with caution; this is a relaxation of placement frequency, not a removal of the disclaimer itself.
Not all experts view this relaxation as positive. Pieter Cohen, M.D., Associate Professor of Medicine at Harvard Medical School expressed concern to Nutraceutical Business Review, warning that reducing disclaimer visibility could mislead consumers. "Then you start saying things such as, ‘We only need it on the actual bottle.’ Then you let the print get smaller," Cohen noted, highlighting the tension between industry simplification and consumer protection.
Durbin Reintroduces the Dietary Supplement Listing Act
While the FDA offers potential labeling flexibility, Congress is considering increased oversight. On Jan. 17, 2026, Senator Dick Durbin reintroduced the Dietary Supplement Listing Act, aimed at modernizing FDA oversight through Mandatory Product Listing (MPL).
The core of the bill would require manufacturers to register products with the FDA, providing product names, ingredient lists, electronic copies of labels, allergen statements, and structure/function claims. This data would populate a public database accessible to consumers.
Senator Durbin’s argument is rooted in the growth of the sector. When DSHEA passed in 1994, there were approximately 4,000 supplements on the market. Today, the FDA estimates there are over 100,000. Durbin argues that the FDA cannot effectively regulate a market it cannot track. "FDA—and consumers—should know what dietary supplements are on the market and what ingredients are included in them. This is FDA’s most basic function," Durbin stated.
As reported by RiverBender, the bill has garnered endorsements from the American Medical Association, US Pharmacopeia, and Consumer Reports. However, the industry itself remains divided, illustrating a strategic difference between its two major trade associations.
A House Divided: CRN vs. NPA
The reintroduction of the Listing Act has reignited a debate between the Council for Responsible Nutrition (CRN) and the Natural Products Association (NPA).
The CRN supports the legislation, viewing transparency as a path to legitimacy and consumer trust. Steve Mister, President and CEO of CRN, stated, "In an era when the Administration has rightly called for more transparency about what we eat and how food is made, it makes sense to apply that same transparency to dietary supplements." The CRN views the registry as a tool to distinguish legitimate, responsible brands from "fly-by-night" actors selling tainted products, arguing that a federal registry is "a transparency tool—not a barrier to innovation."
Conversely, the NPA opposes the bill. Daniel Fabricant, Ph.D., President and CEO of NPA, characterizes it as unnecessary bureaucracy that burdens lawful companies while failing to stop bad actors. Fabricant argues that DSHEA already gives the FDA ample authority; the agency simply fails to use it.
As detailed in Nutrition Insight, NPA fears that the FDA could use the list to arbitrarily challenge ingredients, citing the recent (and reversed) attempt to ban NMN (nicotinamide mononucleotide) as an example of regulatory overreach. "This proposal will hand bureaucrats new leverage over lawful products, cool innovation, and punish companies investing in new science," Fabricant warned.
Conclusion: The Transparency Mandate
As the year progresses, the common thread connecting the Natural Grocers victory and the Durbin bill is transparency. In the food aisle, the courts have ruled that accessibility is key—labels must be readable without a smartphone and use terms the public understands. In the supplement aisle, the debate continues over whether transparency requires a federal database of every product on the market.
For business leaders, the takeaway is operational agility. Packaging workflows must be adaptable, supply chain documentation must be robust, and regulatory monitoring must be constant. The "clean label" trend is extending beyond ingredients to include the regulatory integrity of the package itself.
Natural Grocers has signaled it will remain active, with executive Heather Isely stating, "Natural Grocers will remain actively engaged in the GMO regulatory process." Brands wishing to remain on the shelves of such high-standard retailers must ensure their transparency efforts meet these rising expectations.
Steven Hoffman is Managing Director of Compass Natural Marketing, a strategic communications and brand development agency serving the natural and organic products industry. Learn more at www.compassnatural.com.
Can Trump’s Support Move the Needle on CBD?
Could President Donald Trump's recent endorsement of hemp-derived CBD products provide new momentum for an industry that has struggled in recent years under a patchwork of inconsistent state and federal regulations?
This article first appeared in the November 2025 issue of Presence Marketing’s newsletter.
By Steven Hoffman
In what industry observers have called a surprise move, on Sept. 28, President Donald Trump posted a video on his Truth Social platform promoting the health benefits of cannabinoids, suggesting that covering hemp-derived CBD under Medicare would be a “game changer” and “the most important senior health initiative of the century.” At a time when some members of Congress are pushing for policy changes that could upend the CBD market, Trump’s implied endorsement of CBD is remarkable.
Trump’s post supporting Medicare coverage for CBD products sparked a 36% rise in publicly traded cannabis stocks in the weeks that followed, Yahoo Finance reported. The post also raised hopes that the White House might take a more permissive approach to marijuana regulation following Trump’s statement in August that his administration was exploring a potential reclassification of marijuana — an effort originally proposed under the Biden administration. Removing cannabis from its Schedule I status would mean the federal government acknowledges the plant’s medicinal value.
“I’ve heard great things having to do with medical, and I’ve [heard] bad things having to do with just about everything else,” Trump said during an Aug. 11 White House press conference. “But medical and for pain and various things, I’ve heard some pretty good things.”
The video Trump shared was produced by The Commonwealth Project, an organization dedicated to improving health and longevity for older Americans. It was founded by Howard Kessler, a billionaire and philanthropist with ties to the CBD industry and a longtime friend of Trump’s. According to Independent Voter News, Kessler believes “that medical cannabis could be harnessed to not only provide older Americans with an alternative to traditional prescription painkillers but to reduce soaring health care costs saddling millions of seniors.”
In the video promoted on Truth Social, CBD was described as a way to "revolutionize senior healthcare" by helping reduce disease progression. The narration claimed CBD could help “restore” the body’s endocannabinoid system and ease pain, improve sleep and reduce stress in older adults. It also cited a Fox News segment referencing a Price Waterhouse Coopers report that estimated potential cost savings of “$64 billion a year if cannabis is fully integrated into the healthcare system.”
A Boston- and Palm Beach, Florida-based entrepreneur and philanthropist, Kessler founded Kessler Financial Services, which helped pioneer affinity credit cards. He later obtained one of Massachusetts’ first medical marijuana licenses in 2014, and became the state’s first recreational seller before his company was acquired by a Georgia cannabis firm in 2019. In June 2024, Kessler appeared on Fox News to discuss his efforts to integrate medical cannabis into traditional health care for seniors.
Regulatory Confusion Hinders CBD Market
Regulations around the commercial use of hemp and CBD were significantly eased across the U.S. when industrial hemp was legalized under the 2018 Farm Bill during Trump's first term. However, since its passage, a growing number of state-level battles and lawsuits have emerged regarding the definition of hemp, the “intoxicating hemp” loophole around hemp-derived Delta-8 THC, and the lack of consistent federal and state regulatory frameworks for the cultivation, manufacture, marketing and sale of hemp-derived cannabinoids such as CBD, according to national law firm Buchanan Ingersoll & Rooney.
Trump’s implied endorsement of CBD comes as a bipartisan group of lawmakers pushes back against attempts to ban hemp-derived THC products, arguing that such action would “deal a fatal blow” to the hemp industry and violate congressional rules. In a letter sent to House Speaker Mike Johnson (R-LA) on Sept. 26, House Oversight and Government Reform Committee Chairman James Comer (R-KY) and 26 other members warned that appropriations legislation containing hemp ban provisions would devastate the industry that emerged after hemp’s 2018 legalization.
A group of eight Democratic senators also sent a letter in September urging leadership to pursue regulation rather than prohibition, warning that banning products containing any amount of THC would trigger major upheaval in the hemp market. (Under the 2018 Farm Bill, hemp is legally defined as containing no more than 0.3% THC on a dry weight basis.) Meanwhile, dozens of hemp farmers from Kentucky have urged Senate Minority Leader Mitch McConnell (R-KY) to back away from efforts to re-criminalize certain hemp-derived products, Louisville Public Media reported.
Kentucky Sen. Rand Paul also warned that the cannabis policy movement has “swung hard on the prohibitionist side.” In June, he introduced the Hemp Economic Mobilization Plan (HEMP) Act to counter potential restrictions, proposing to triple the amount of THC allowed in hemp while addressing several other regulatory challenges facing the industry.
For its part, the U.S. Food and Drug Administration (FDA) reaffirmed in January 2020 that it is unlawful to introduce food containing added CBD into interstate commerce, or to market CBD as, or in, dietary supplements. Now, according to Marijuana Moment, while Trump was endorsing CBD on Truth Social, the FDA quietly updated its adverse drug event reporting forms to track incidents related to hemp-derived cannabinoids, including CBD — part of an effort to gather more data on potential health effects associated with such products.
Hemp Industry Responds to President’s Support
In an Oct. 7 letter to President Trump, Jonathan Miller, legal counsel for the U.S. Hemp Roundtable, praised the president’s acknowledgment of hemp’s potential and urged him to oppose the proposed hemp ban:
"The recent video you shared about the extraordinary value of hemp products was important, raising awareness on the positive impact our American-grown and manufactured products have. Here at the U.S. Hemp Roundtable, our members are focused on giving Americans choices in improving their overall health and wellness ... but now we need your help! Congress is close to passing a hemp ban, reversing the work you led in 2018 to make hemp blossom. A proposed definition change to hemp, being touted as protecting Americans, would wipe out 95% of this uniquely American industry that you are so proud of,” Miller stated.
He continued: “A more effective way to protect American consumers and jobs would be to support and demand robust hemp regulation — age restrictions along with uniform testing, labeling, and packaging requirements. Outright prohibition is not the answer, nor would it make anyone safer. Banning legal hemp products that are already regulated at the state level will not protect consumers; it would only shift hemp to the black market and destroy a rising American industry in the process. ... A ban would put American farmers, American businesses, American consumers, our veterans, seniors, and more than 328,000 American workers at risk."
Miller added that "American voters are on your side on this issue. In Texas, a state with a rapidly growing hemp market, 76% of your voters and 78% of seniors favor legal, regulated hemp sales. In fact, more than 62% of Texans say they are more likely to support candidates who back the regulated sale of hemp-derived products."
Bottom line: Trump’s apparent support for CBD could mark a turning point for a sector long constrained by legal uncertainty. Whether the endorsement leads to meaningful policy change remains to be seen — but it has already reignited momentum, investment, and public discourse around hemp-derived wellness products in America’s fast-evolving natural health market.
Steven Hoffman is Managing Director of Compass Natural Marketing, a strategic communications and brand development agency serving the natural and organic products industry. Learn more at www.compassnatural.com.
EWG Publishes ‘Clean 15’ to Reduce Dietary Pesticide Exposure; Study Shows Glyphosate Still Carcinogenic at Levels Deemed Safe
This article first appeared in the July 2025 issue of Presence Marketing’s newsletter.
By Steven Hoffman
As public awareness of the connection between food and health continues to grow, so too does concern over pesticide residues in the food we eat every day. This month, two major developments underscore just how crucial it is to pay attention not only to what’s on our plates — but how it got there.
On June 11, the Environmental Working Group (EWG) released its 2025 Shopper’s Guide to Pesticides in Produce, including the highly anticipated “Clean Fifteen” and “Dirty Dozen” lists. These guides rank popular fruits and vegetables based on pesticide contamination, using data compiled from the USDA and FDA. For nearly two decades, these rankings have helped consumers make more informed decisions about when buying organic matters most.
This year’s “Clean Fifteen” offers some good news: Nearly 60% of the tested samples of the 15 least-contaminated conventional produce items showed no detectable pesticide residues whatsoever. Topping the clean list were avocados, sweet corn, pineapple, onions, and papaya — all relatively safe bets for budget-conscious shoppers looking to reduce pesticide intake without going fully organic.
But the release of EWG’s guide was accompanied by sobering news on another front: The results of a two-year study published in Environmental Health adds to a growing body of evidence suggesting that glyphosate — the active ingredient in Bayer-Monsanto’s Roundup®, the world’s most widely used herbicide — may cause multiple types of cancer, and at doses considered safe by regulators.
“Our study provides solid and independent scientific evidence of the carcinogenicity of glyphosate and glyphosate-based herbicides,” said lead investigator Daniele Mandrioli of the Ramazzini Institute in Italy.
Glyphosate and the American Diet
Glyphosate has been a mainstay of industrial agriculture since the 1970s, praised for its broad-spectrum weed-killing power. With the rise of genetically modified crops engineered to resist glyphosate, its use exploded in the late 1990s and 2000s. Today, it’s sprayed on millions of acres of GMO corn, soybeans, cotton, and canola. But it doesn’t stop there.
In conventional grain production, glyphosate is also used as a desiccant — sprayed just before harvest to dry out crops like oats, wheat, lentils, and chickpeas. That means it doesn’t just show up in livestock feed. It ends up in our breakfast bowls and lunchboxes: in oatmeal, crackers, tortillas, hummus, and cereal — it can even end up in products marketed as “natural” or “healthy.”
Glyphosate is now so prevalent in our environment that it has been detected in everything from rainwater to breast milk. A 2022 CDC study found glyphosate in the urine of 80% of a representative sample of U.S. children and adults. Just last year, EWG reported that popular oat-based cereals and snack bars still contained detectable glyphosate residues, years after promising to reformulate.
So, how dangerous is it?
A Closer Look at the Science
The new Environmental Health study, published in June 2025, evaluated nearly 2,000 previously published studies to reassess glyphosate’s potential health risks. It concluded that even very low doses — far lower than currently allowed by regulatory agencies — can pose significant risks of cancer, hormone disruption, reproductive harm, and immune suppression. The researchers found that glyphosate can interfere with endocrine signaling pathways at parts-per-billion levels, meaning even tiny exposures could be biologically active.
This follows a 2015 determination by the World Health Organization’s International Agency for Research on Cancer (IARC), which classified glyphosate as a “probable human carcinogen.” Since then, thousands of lawsuits have been filed against Bayer-Monsanto, many resulting in high-profile jury verdicts linking glyphosate exposure to non-Hodgkin’s lymphoma. Although Bayer continues to deny glyphosate’s carcinogenicity and has spent billions to settle lawsuits, public confidence in the safety of this chemical is eroding.
And now, with researchers warning there may be no safe level of exposure, the need for regulatory reassessment — and consumer action — is more urgent than ever.
What the ‘Clean Fifteen’ Tells Us — and What It Doesn’t
In the midst of all this, EWG’s Shopper’s Guide to Pesticides in Produce offers a valuable, practical resource for consumers trying to navigate the complexity of the modern food system.
EWG analyzed over 47,000 samples of 46 popular fruits and vegetables. The “Clean Fifteen” list identifies produce items that typically have the lowest pesticide levels, even when grown conventionally. This year’s top 15 are:
Avocados
Sweet corn
Pineapple
Onions
Papaya
Frozen sweet peas
Asparagus
Honeydew melon
Kiwi
Cabbage
Watermelon
Mushrooms
Mangoes
Sweet potatoes
Carrots
It’s worth noting that some of these crops, such as papaya and sweet corn, are frequently genetically modified. That means they may be lower in pesticide residues, but still part of the chemical-dependent industrial agriculture model.
In contrast, the “Dirty Dozen” — which includes strawberries, spinach, and kale — are best purchased organic due to their high pesticide loads. For instance, 90% of strawberry samples tested had detectable pesticide residues, and spinach samples had, on average, 1.8 times more pesticide residues by weight than any other crop.
The takeaway: if you can’t afford to buy everything organic, prioritize organic options for items on the Dirty Dozen, and rest a bit easier when purchasing from the Clean Fifteen.
Resources & References
EWG Shopper’s Guide to Pesticides in Produce: https://www.ewg.org/foodnews/
Glyphosate cancer study summary: https://www.thenewlede.org/2025/06/new-study-adds-to-evidence-that-glyphosate-weed-killer-can-cause-cancer/
Steven Hoffman is Managing Director of Compass Natural Marketing, a strategic communications and brand development agency serving the natural and organic products industry. Learn more at www.compassnatural.com.
DOGE Days: From Supplements to Organic Farming, Natural Industry Feels Cuts
By Steven Hoffman
As the Department of Government Efficiency, or DOGE, led by billionaire Elon Musk under the administration of President Donald Trump, cuts jobs and funding for programs at U.S. agencies across the board, natural and organic food and dietary supplement producers are feeling the impact of DOGE cuts to FDA, USDA, USAID and others.
Cuts to FDA have resulted in the resignation of James Jones, FDA’s top official in charge of food safety and nutrition, following what he called “indiscriminate” layoffs of dozens of food safety inspectors. Jones, who joined the agency in 2023, said the cuts would make it “fruitless” to continue his role. “I was looking forward to working to pursue the department’s agenda of improving the health of Americans by reducing diet-related chronic disease and risks from chemicals in food,” Jones wrote. News of the resignation was first reported on Feb. 17 by FoodFix.
The U.S. Department of Health and Human Services announced plans in mid-February to fire 5,200 probationary employees across its agencies, including the National Institutes of Health (NIH), the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). The layoffs appeared to focus on employees in the agency’s departments for food, medical devices and tobacco products. It was not clear whether FDA employees who review drugs were exempted. However, Fierce Healthcare reported on Feb. 22 that hundreds of fired FDA probationary workers have received notices from the agency that their terminations were rescinded.
Following news of the DOGE’s layoffs at the FDA, including a number of staff firings at FDA’s office of Dietary Supplement Programs, the Council for Responsible Nutrition expressed concern about the FDA’s ability to effectively oversee dietary supplements and food safety. “As the FDA deputy commissioner steps down, it’s critical that the agency maintains adequate staffing and expertise to uphold consumer confidence in the food supply,” CRN said in a statement.
“While staffing changes can occur during any presidential transition, it is critical that the FDA maintains the resources, expertise and staffing levels necessary to ensure effective dietary supplement oversight that undergirds consumer confidence in the supplement market,” said Jeff Ventura, CRN’s VP of Communications.
USDA Pauses Two Major Organic Programs
At USDA, pauses and cuts in funding for organic transition and soil conservation programs are leaving farmers on the hook for millions of dollars they invested on the promise of reimbursement, while “accidental” firings of bird flu researchers at the agriculture agency have exacerbated the high price and limited availability of eggs. (According to USDA data, the price of eggs increased 38.2% since the beginning of 2025, with the cost of a dozen conventionally produced eggs exceeding of $8 in February 2025.)
Federal officials also are withholding funding for two major organic agriculture programs that make payments directly to farmers, jeopardizing millions of dollars in funding just ahead of the 2025 planting season, reported E&E News by Politico on February 6. “The pause on the $85 million Organic Market Development Grant program and the $100 million Transition to the Organic Partnership Program has jolted farmers, nonprofits and businesses struggling to make planting and hiring decisions. Even if the pause on funding is lifted, it could put farmers out of business,” wrote E&E News reporter Marcia Brown. According to Brown, USDA has yet to release funding for the programs, even though federal courts ordered an end to the across-the-board freeze.
In addition, USDA’s Supplemental Nutrition Assistance Program (SNAP), formerly known as the Food Stamp program, is under DOGE scrutiny, plus the House of Representatives budget plan seeks to cut up to $230 billion from SNAP. Such funding cuts would affect sales for natural and organic food producers, including for such staple healthy products as organic dairy and plant-based foods that are frequently purchased by SNAP recipients.
According to Georgie Lee Smith, who blogs under the moniker Farmer Georgie, the loss of scientists and researchers at USDA agencies including the Agricultural Research Service (ARS) and the Animal & Plant Health Inspection Service (APHIS) will have a long-term negative effect on the ability of U.S. agriculture to respond to issues and opportunities, and maintain its position as one of the world’s leading food producers.
Beginning in mid-February, “DOGE took aim at the United States Department of Agriculture, aka the USDA, firing ‘probationary’ employees in what has been described as a ‘blood bath’ of terminations. To be clear, probationary periods can run from one year to three, depending upon the job, and apply even to long-time USDA employees who have recently taken a job promotion. Translation — it’s a lot of folks, many with significant dollars invested into their training,” Smith wrote.
“The researchers and scientists at ARS are on the frontline of nutrition, food safety and quality, livestock and crop production, natural resources and sustainable agricultural systems. Along with our land grant university systems, ARS researchers are often the first area of investment into solving critical food and agricultural issues, whether that’s preventing food-borne illness outbreaks to breeding more climate-resilient crops and livestock to new ways to combat pests and diseases impacting food production,” she wrote. Smith also pointed to the risks associated with cutting staff at APHIS, USDA’s agency responsible for combatting the escalating avian flu crisis.
“Many farmers and ranchers are still holding onto hope that the funding freezes that have their grants and cost-share monies tied up are only a bump in the road. Perhaps these USDA terminations will be the same. But I’m afraid that is not the case,” Smith wrote on February 16. “Secretary (of Agriculture) Brooke Rollins issued a press release … that DOGE had terminated 78 USDA contracts totaling more than $132 million, with more than 1,000 contracts still under review. And DOGE tweeted they had eliminated an $8.2 million USDA contract to implement programs administered under Biden’s climate-smart initiative, which had funneled $3 billion in grant funds to agricultural producers, marketing organizations and forest landowners nationwide to support the adoption of climate-smart practices. Again, I can’t help but point out that the large majority of the climate-smart grants specifically supported the same ‘soil health/regenerative ag’ mantra that RFK Jr. is purporting. I feel like we just cut off our noses to spite our faces,” Smith commented.
Dismantling of USAID Impacts U.S. Farmers
For the U.S. agriculture economy, 40% of government food assistance comes from American farms through programs including the U.S. Agency for International Development (USAID), which purchased $2 billion in food from American farmers in 2024. As such, the dismantling of USAID, along with its Food for Peace program, has eliminated a valuable market for farmers. It also has resulted in nearly $500 million worth of donated food sitting unused or rotting on docks and in warehouses, according to a report issued on February 10 by the Inspector General of USAID.
“Should parts of USAID be reformed or revisited? Certainly. But shutting down the entire agency in less than two weeks is not the way to do it,” wrote Erin Sikorsky, Director of the Center for Climate and Security and former aide to Wisconsin Congressman Ron Kind, in the Feb. 17, 2025, edition of the Milwaukee Journal Sentinel.
“I know Wisconsinites are proud of the role our farm industry plays in supporting food security and preventing starvation worldwide. The Wisconsin Congressional delegation has consistently stood up for Wisconsin farmers, and they must do the same today by opposing this reckless destruction of these life-saving and economically important food aid programs. This action is a reversal of decades of bipartisan support for programs that provide global food aid and prevent starvation, precisely because such programs help American farmers, help those most in need, and prevent conflict and instability that threatens our national security,” Sikorsky wrote.
Steven Hoffman is Managing Director of Compass Natural, providing public relations, brand marketing, social media and strategic business development services to natural, organic, sustainable and hemp/CBD products businesses. Contact steve@compassnaturalmarketing.com.
Presence MarketWatch 2025
This article first appeared in the January 2025 issue of Presence Marketing’s newsletter.
By Steven Hoffman
With the Trump administration returning to the White House and the GOP controlling both the Senate and House of Representatives by narrow margins, the year 2025 is sure to bring significant change to regulatory policy, business and the economy, not just for the U.S. but also the world. To help leaders in the natural channel navigate the opportunities and challenges ahead, Presence Marketing will track and report on these issues over the course of the year ahead. Read on for a snapshot of some of the major issues that will impact the natural, organic and nutritional products market over the coming year.
Tariffs and Food Prices
President-elect Donald Trump ran on a campaign to lower grocery prices, which rose 23% since the onset of the Covid pandemic in Spring 2020. Food inflation has slowed over the past year, according to NBC News, and is now less than 2% as energy prices and supply chains have stabilized. Yet, experts caution that a combination of tariffs and mass deportations could have a further destabilizing effect on agriculture, food production and grocery prices. Trump has threatened to impose tariffs up to 60% on goods from China, and a 25% tariff on products from Mexico and Canada – all countries that are significant exporters of food and other products to the U.S. market.
In a Time Magazine interview in December 2024, Trump acknowledged it may be difficult to bring down grocery prices, saying, “Look, they got them up. I’d like to bring them down. It’s hard to bring things down once they’re up.” According to a study from the Peterson Institute for International Economics, Trump’s proposed tariffs on Mexico and Canada would have the biggest impact on prices for autos, vegetables, fuel, prepared food and animal products, reported CNN Business. The U.S. relies on Mexico for 89% of its imported avocados and 91% of foreign-grown tomatoes. “Higher tariffs on Mexico and Canada will … put upward pressure on U.S. food prices,” the Peterson Institute said. While it’s too soon to determine whether Trump will actually impose tariffs or if trade agreements can be reached to prevent them, “The only certainty is that new tariffs will be costly for the United States,” said the Peterson Institute study’s authors.
Food, Farm Workers and Mass Deportation
California’s Monterey County is the fourth-largest crop-producing county in the nation, with the agriculture industry there contributing $4.4 billion to the economy, and with an estimated 55,000 farm workers, including many who are undocumented. As such, the area’s growers have expressed concern that much of their workforce could disappear as a result of potential mass deportations once the Trump administration takes office. In an interview on Dec. 19, 2024, with NBC Bay Area News, Monterey County Farm Bureau CEO Norm Groot said, “It will absolutely impact food prices at the consumer level. If it impacts local and nationwide supplies, that will have a price increase.” NBC reported the farm bureau is teaming up with county officials and other stakeholders to create a task force in addressing local concerns around mass deportations, including concerns around family and child separation. "It's interesting that four years ago during the pandemic, they were essential," Groot said. "And now all of a sudden we’re looking at it from a different perspective and trying to understand how that dynamic has changed."
And it’s not just Monterey County – while it’s estimated that undocumented workers make up only 5% of the total U.S. workforce, the share of undocumented workers across the nation’s food supply chain is at least 16%, reported Successful Farming. In some industries this number is higher – the Idaho Dairymen’s Association estimated that nearly 90% of the state’s dairy workers were born outside of the U.S. According to a September 2024 study by the Peterson Institute, mass deportation could lead to a 10% increase in food prices. Between higher food prices that could come with proposed tariffs – and potential government bailouts funded by U.S. taxpayers to provide assistance to farmers affected by deportations – Americans could potentially get “double-whammied” by the higher costs and supply chain disruptions these proposed policies could bring.
RFK, FDA and the Nation’s Health
MAHA has become a rallying cry for many in the natural health and nutritional supplements industry as Congress weighs the nomination of Robert F. Kennedy, Jr. Kennedy, a lawyer, environmentalist and controversial health advocate, is Trump’s pick to lead the U.S. Department of Health and Human Services (HHS), a Cabinet-level position that oversees the U.S. Food and Drug Administration (FDA), Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), and others.
On one hand, RFK’s team is weighing a rewrite to the FDA’s rules overseeing food additives and taking a hard look at the harmful chemicals and pesticides used in food production. On the other hand, RFK’s top lawyer Aaron Siri stirred controversy when it was reported in December 2024 by CNN and others that he had petitioned the FDA to revoke approval of the polio vaccine. The World Health Organization declared that polio was eradicated in 2019 but warned it could re-emerge if vaccination coverage declines. According to a Dec. 4, 2024, article in Forbes, Kennedy criticized the FDA in a post on X (formerly Twitter) for “suppressing” a wide range of items, including “psychedelics, peptides, stem cells, raw milk, hyperbaric therapies, chelating compounds, ivermectin, hydroxychloroquine, vitamins, clean foods, sunshine, exercise, nutraceuticals, and anything else that advances human health and can’t be patented by Pharma.”
Kennedy will have an ally in Martin Makary, M.D., a surgeon, public policy researcher at Johns Hopkins University and a member of the National Academy of Medicine, and President-elect Trump’s choice to serve as FDA Commissioner. In September 2024, Makary joined RFK at a round table in Congress on health and nutrition, where he criticized how food in the U.S. is grown and processed. "We have poisoned our food supply, engineered highly addictive chemicals that we put into our food. We spray it with pesticides that kill pests. What do you think they do to our gut lining in our microbiome?" Makary said. In related news, Trump’s pick for Surgeon General, Dr. Janette Nesheiwat, a family medicine doctor who runs a chain of urgent care clinics in New York, was a regular Fox News contributor and is an advocate for nutritional supplements, marketing her own brand of dietary supplements called BC Boost, containing vitamins C, B-12, D and Zinc.
Brooke Rollins Nominated to Lead USDA
President-elect Trump in November nominated Brooke Rollins, President and CEO of the America First Policy Institute, a conservative think tank based in Texas, to lead the U.S. Department of Agriculture. “As our next Secretary of Agriculture, Brooke will spearhead the effort to protect American farmers, who are truly the backbone of our country,” Trump said in a statement. Rollins is a graduate of Texas A&M University, with an undergraduate degree in agriculture development. “From her upbringing in the small and agriculture-centered town of Glen Rose, Texas, to her years of leadership involvement with Future Farmers of America and 4H, to her generational family farming background, to guiding her four kids in their show cattle careers, Brooke has a practitioner’s experience, along with deep policy credentials in both nonprofit and government leadership at the state and national levels,” the statement said.
“We congratulate Brooke Rollins on her nomination as Secretary of Agriculture. This is an important moment for U.S. agriculture, and we are optimistic about the opportunities her leadership will bring to rural America,” Amy France, chairwoman of the National Sorghum Producers in Scott City, KS, told Successful Farming. "Sorghum farmers are at the forefront of innovation, contributing to domestic biofuels and heart-healthy, nutritious, ancient grain foods. We are eager to work with her to advance policies that strengthen the sorghum industry and benefit growers nationwide.”
“The Department of Agriculture plays a pivotal role in safeguarding our food supply, addressing food insecurity, managing our forests, as well as supporting America’s farmers and rural communities who are on the frontlines of the climate crisis,” said Rebecca Riley, Managing Director, Food and Agriculture, for the Natural Resources Defense Council (NRDC). “Rollins needs to invest in America’s farmers – from small family farms to larger-scale operations – and to work toward a resilient and equitable food system that puts healthy food on the table, restores our soil, protects the climate, and safeguards the health of our communities … now is not the time to undermine climate-smart farming practices, favor industrial agriculture at the expense of small producers and consumers, or gut the nutrition programs that many Americans rely on,” Riley said.
California’s AB 660 Sets Landmark Food Date Labeling Standards
California Governor Gavin Newsom in September 2024 signed into law the nation's first mandatory food date labeling reform bill. California Assembly Bill 660 (AB 660) standardizes the use of “Best If Used By” and “Use By” dates on food labels, and prohibits the use of “Sell By” dates. The new law requires manufacturers to use the same phrase for date labels across their products, reported Food Safety. Beginning July 1, 2026, companies selling food products in California must only use “Best If Used By” to indicate the date by which a product will reach its peak quality, and “Use By” to indicate the date by which a product’s safety can no longer be guaranteed. The use of consumer-facing “Sell By” dates will be prohibited to reduce the chances of consumers confusing “Sell By” dates with quality or safety dates.
“On grocery store shelves today, there are more than 50 differently phrased date labels on packaged food. Some phrases are used to communicate peak freshness of a product or when a product is no longer safe to eat. Others, like ‘Sell By,’ are used only to inform stock rotation in stores but mislead some consumers into thinking the product is no longer safe to eat. AB 660 will close this gap by requiring manufacturers to use the same phrase for date labels across their products,” NRDC said in a statement.
Of course, as goes California, so goes the country. “AB 660 is game changing, not just for California, but for the country. It will be the first law of its kind to end the ridiculous confusion that causes consumers to throw out almost $15 billion of perfectly good food nationwide. It will also help reduce the significant toll that wasting food has on our planet,” Dana Gunders, President of reFED, told BioCycle Magazine. “Having to wonder whether our food is still good is an issue that we all have struggled with. Today’s signing of AB 660 is a monumental step to keep money in the pockets of consumers while helping the environment and the planet,” said Assemblymember Jacqui Irwin, author of the bill.
Steven Hoffman is Managing Director of Compass Natural, providing public relations, brand marketing, social media and strategic business development services to natural, organic, sustainable and hemp/CBD products businesses. Contact steve@compassnaturalmarketing.com.
After Boom and Bust, Could Hemp Market Be Stabilizing?
This article first appeared in the May 2024 issue of Presence Marketing’s newsletter.
By Steven Hoffman
Could the hemp industry be on the cusp of a turnaround? After several years of volatility for U.S. hemp growers, prices and acreage in many states are beginning to stabilize or are rising modestly, according to the National Hemp Report, published by the U.S. Department of Agriculture (USDA) in April 2024.
According to the USDA, the value of U.S. hemp crop production in 2023 totaled $291 million, up 18% from 2022. Based on a survey sent out in January 2024 to producers across the country as part of USDA’s national agriculture census, the report shows signs of renewed market growth and improved on-farm efficiencies. “There was the big boom, then the big falloff, and this year everything is sort of leveling out,” Joshua Bates, a USDA statistician who wrote the landmark National Hemp Report, told MJBiz Daily.
“The hemp industry is stabilizing into its various categories including: 1) cannabinoids, nutraceuticals and flower; 2) food, feed and nutrition; and 3) fiber and industrial materials and applications. At this time, a much clearer picture is emerging as to where these categories can end up over the next three, five and ten years,” said Morris Beegle, publisher of Let’s Talk Hemp and producer of NoCo Hemp Expo, the leading trade show and conference for the hemp industry.
“The past several years posed significant challenges, starting with the pandemic’s onset and exacerbated by the federal government’s regulatory ambiguity regarding CBD and hemp-derived cannabinoids. Add to that burdensome regulations imposed on farmers endeavoring to cultivate hemp fiber and grain – crops that deserve equitable treatment akin to any other commodity. Such circumstances deter participation in this budding industry. What we truly require are coherent federal regulations applicable universally, instead of disjointed state-level initiatives that render the industry vulnerable and unsupported,” Beegle said.
Recovery on the Horizon
Production of industrial hemp for food, fiber and flower took off after it was legalized in the 2018 federal Farm Bill. In the years before the Covid pandemic, hemp acreage was on the rise, entrepreneurs and investors flocked into the market, and there was an explosion in the number of CBD and other hemp-related brands.
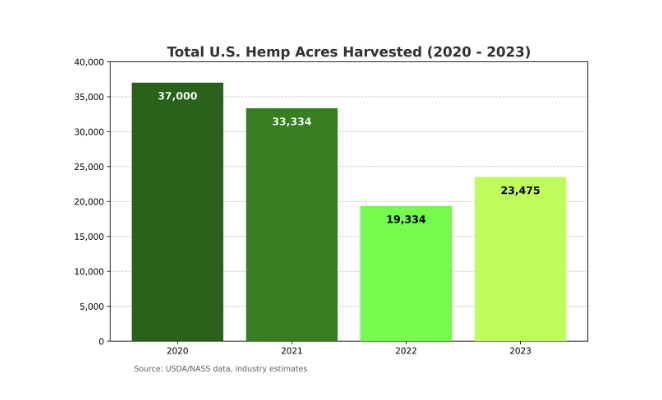
However, since 2020, the market for products made from hemp (defined as containing less than 0.3% THC) experienced a significant downturn, sparked by the pandemic and made worse by a lack of regulatory consistency over CBD and other hemp-derived cannabinoid compounds from the U.S. Food and Drug Administration (FDA) and state regulators. Investors pulled back. Brands did everything they could to survive; some didn’t. Overproduction during this time, too, led to steep drops in the price of hemp. As a result, hemp acreage plummeted from an estimated peak of 37,000 acres harvested in 2020 to a little more than 19,000 acres harvested in 2022.
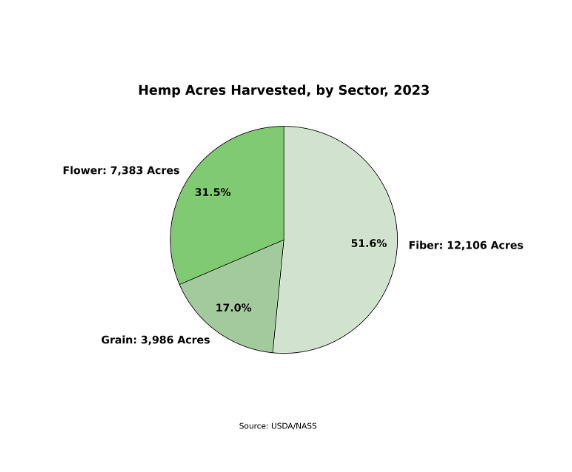
Now, according to USDA’s hemp report, overall hemp acreage harvested in 2023 increased 21% to a total of 23,475 acres, signaling a potential turnaround in the market.
Hemp flowers for CBD and other hemp-derived cannabinoids -- including the intoxicating compounds Delta 8 and Delta 9 – dominated the market in 2023. While flower acreage remained relatively flat at approximately 7,000 acres in 2023, flower producers experienced an income gain of 35%, according to USDA data. Of the $291 million in market value for hemp in 2023, $241 million of that income came from hemp flowers. However, based on regulatory and safety concerns around Delta 8 and other hemp-derived psychoactive compounds, a number of industry observers feel this growth may be unsustainable, as lawmakers could severely limit the sale of these products.
Speaking of the hemp flower sector, economist Beau Whitney of Whitney Economics said, “It is quite possible that the cannabinoid industry has peaked. The excess inventories are mostly depleted and there is not enough acreage, in our view, to backfill with enough supply to sustain the level of sales experienced currently. This is a mistake by legislatures and regulators and now opens the door up for cheaper, but potentially lesser quality imports,” he cautioned.
Hemp grown for fiber – used in textiles and industrial applications – accounted for 52% of all hemp acreage harvested in 2023. While acreage grew, USDA reported that prices dropped and farmers brought in less income. However, says Whitney, “Hemp fiber is where we are forecasting significant growth in the short term, while cannabinoids and grains get sorted out. Hemp for plastics and automotive are already established in the global marketplace and are expanding in the United States. Hemp as a construction material is also increasing, but that growth has been somewhat sluggish in its initial ramp-up.”
Regarding hemp grown for food and grain, Whitney remarked, “The food industry is pretty steady. One major game changer would be FDA approval for hemp grains as an animal feed. It appears that the FDA is setting up unrealistic requirements for cannabinoid content in feed, so much so that consumers/animals would have less strict requirements for heavy metals and poisons than CBD content. There is a huge market for hemp grain internationally, but other supply chain issues and geopolitical tensions are impacting expansion efforts by U.S. hemp operators.”
According to USDA’s National Hemp Report, hemp grown for grain accounted for 17% of all hemp acreage harvested. Canada and China remain the world’s leading countries for hemp grain production, yet, as plant-based foods become more popular, U.S. growers stand to benefit from opportunities in growing hemp for food, as it is one of the richest plant-based sources of protein, essential fatty acids and other key nutrients.
“The use of hemp in automotive, pulp and paper (packaging), and building material applications are all increasing in the pace of growth,” Whitney added. “The major headwind is the narrative that hemp is a drug. The lack of awareness and education by policymakers on the potential of hemp is its limiting factor. Policymakers are using a sledgehammer instead of a scalpel when making hemp policy, and it’s having a ripple effect throughout the industry from an operator, investor and infrastructure perspective,” he said.
Stuck Between FDA and the MJ Industry
“There are two big battles going on in Washington, DC, right now. One is the hemp industry against the FDA. The other is hemp vs. marijuana,” observed Jonathan Miller, General Counsel of the U.S. Hemp Roundtable, the hemp trade’s leading advocacy and lobbying group.
“We’ve been trying to get the FDA to regulate CBD and other extracts. First, they said they were working on it and now they say they need congressional authority. It’s a game of pointing fingers – Congress says it needs guidance from FDA, and FDA says it needs direction from Congress, so we’ve got a stalemate. However, there has been a lot of congressional and public pressure on FDA to act, so that may be a reason why the agency is talking to us now,” Miller said. “We would love to see something resolved this year; there is talk of an interim step where Congress could potentially pass a law to validate existing state programs in the absence of consistent federal regulations. The Farm Bill may not happen until next year; meanwhile our efforts to find a vehicle for this compromise will continue.”
And, Miller said, “As always, we advise companies to act like they are being regulated by the FDA as a dietary supplement or a functional food and beverage product, and to operate within those regulations.”
In referring to competition with operators in the marijuana space, Miller said, “Regarding the marijuana industry, it is not monolithic, but there are a growing number of organizations that have made it their objective to kill the hemp industry as a means to capture competitive gain. This includes ATACH, the American Association for Hemp and Cannabis – though there are no hemp members – and the US Cannabis Council. Both have introduced plans that they would like to see in the Farm Bill to federally criminalize any hemp product that has any level of THC in it. That would criminalize all but CBD isolates,” he said.
“We are fighting both in the public eye and behind the scenes right now, and there’s a big battle going on in California in this regard. This is a threat we have to watch from the left flank. We are hopeful that any effort like this will die in Congress, but we are not banking on it; in fact, we are working very hard to prevent it. Just last week, we met with 55 different members of Congress and staff to discuss these issues and to present the hemp industry's position,” Miller added.
“All sectors of the hemp industry are growing, with the cannabinoid sector growing rapidly, to the point where it is outpacing the marijuana industry,” observed cannabis industry attorney Rod Kight of Kight Law. “The opportunities exist for nimble companies that are willing to take the time to navigate the rapidly evolving regulatory landscape and who can pivot as needed when regulations and markets change. I have hope that hemp can lead the path to true and comprehensive cannabis reform.”
FDA’s Position
When asked if the FDA could envision a scenario in which it would allow the sale of CBD as a dietary supplement without congressional action, Patrick Cournoyer, Senior Science Advisor with the FDA replied with the following:
“The FDA has concluded that the existing regulatory framework for dietary supplements is not appropriate for CBD. Given the available evidence, it is not apparent how CBD products could meet the safety standard for dietary supplements. For example, we have not found adequate evidence to determine how much CBD can be consumed, and for how long, before causing harm. Therefore, we do not intend to pursue rulemaking allowing the use of CBD in dietary supplements.”
Cournoyer’s office referred to FDA’s statement from January 2023, where the agency concluded that “existing regulatory frameworks for foods and supplements are not appropriate for cannabidiol,” and that it will work with Congress on “a new way forward.”
Added Cournoyer, “The FDA supports sound, scientifically based research into the medicinal uses of drug products containing cannabis or cannabis-derived compounds and will continue to work with companies interested in bringing safe, effective, and quality products to market.” Cournoyer referred to guidelines published on FDA’s webpage, “FDA and Cannabis: Research and Drug Approval Process.”
In addition to food, supplements and drugs intended for human consumption, FDA has regulatory oversight of animal feeds. Regarding the use of hemp in animal feed, an FDA spokesperson said, “After comprehensive review of data submitted by a sponsor, in January 2024, the FDA recommended to the Association of American Feed Control Officials (AAFCO) that a proposed ingredient definition for hemp seed meal in the feed of laying hens be included in the AAFCO Official Publication. The FDA has not reviewed any other submissions for the use of hemp seed or hemp seed derived ingredients in animal food.”
Wendy Mosher, VP of the Hemp Feed Coalition, told AgWeb in January 2024 that allowing hemp seed meal into feed mixes for laying hens marks the first hemp feed ingredient to get federal recommendation and interest by AAFCO. “You can’t have a commodity crop without a feed opportunity for that crop,” Mosher said.
In the absence of federal approval of hemp in animal feed, Texas legalized hempseed oil and hempseed meal for chickens and horses in 2023. Kentucky in 2022 allowed a limited amount of hempseed meal and hempseed oil as ingredients in the diets of layer, broiler, and breeder chickens. Montana legalized hemp or hemp-derived products in 2021 for “non-consumption animals,” i.e., pets, specialty pets, and horses, reported Hemp Benchmarks. However, in December 2023, New York Governor Kathy Hochul vetoed bills to allow hemp seed in animal feed, citing the need for more information.
While federal lawmakers and regulators move slowly in allowing the use of hemp in animal feed, USDA is providing grants to universities and others to research the potential use of hempseed, meal and biomass in animal feed. For example, researchers at Prairie View A&M University in Texas in 2023 received a $300,000 grant from USDA to explore hemp as an alternative grain in animal feed.
Building with Hemp
Building with hemp in the United States is increasing year over year, said Jean Lotus, Editor and Publisher of HempBuild Magazine. “Prices on building-grade hemp hurd remain high and the supply chain is neither complete, nor consistent. But hemp building in the United States is capturing the imaginations of developers and motivated owner-builders. Notable projects this year include a multi-unit tip-up hempcrete panel project in Newburyport, MA, and successful attainable hempcrete housing built by the Lower Sioux of Morton, MN,” she said.
“People may say hemp is dying, but it also recently received approval from the IRC (International Residential Codes) for the use of hempcrete in the U.S. for commercial construction,” noted James Johnson, Principal of JJGro in San Antonio, TX. Johnson, who discovered hemp’s healing properties while dealing with PTSD after 21 years of service in the U.S. Air Force, consults with hemp producers throughout the country. “Hemp is starting to be considered as part of the mix in mainstream commercial construction. It’s not going to replace traditional materials, but it will capture between 2% and 5% of the building construction space, Johnson predicted.
Lotus added that research in hemp building materials received significant funding in the past year, including $1.9 million from the U.S. Army; $1.5 million from the Department of Energy; and $1.1 million from the New York State Energy Resource and Development Authority (NYSERTA).
“Meanwhile, the regulatory environment for hempcrete's superior insulation and carbon sequestering properties is improving,” she said. “In April 2024, the U.S. Department of Energy released a roadmap to decarbonize the U.S. building industry. The report, Decarbonizing the U.S. Economy by 2050: A National Blueprint for the Buildings Sector, emphasized building with biogenic, regenerative materials. Hemp-lime and hemp-batt insulation perfectly fit the bill! With new companies developing carbon credits for hempcrete projects, there is more excitement and investment opportunity to come.”
“We are seeing increased interest in hemp across the board from consumers, universities, and large corporations wanting to utilize hemp as an input or ingredient. I think there's opportunities in every sector for savvy, smart business operators. While cannabinoids provide the biggest boom/bust potential, there are long term opportunities in the fiber and grain markets as the regulatory framework within those categories will eventually get to a point of any other commodity crop, for the most part,” said Morris Beegle.
“As to headwinds, it's still all political and bureaucratic,” Beegle added. “The industry needs to be unified in its voice to combat correctly, and I think we have made real progress the last few years with the vast majority of the ‘real’ industry aligning on messaging and action. We’re also seeing progress and persistence of the industry in delivering on the promise of hemp as an alternative resource to many of the world's current and future problems. The future is bright regardless of what some in the media, and some on social media, would like people to believe.”
Save the Date: NoCo Hemp Expo, Aug. 10-12, 2025, Estes Park, CO
U.S. Hemp Leaders Unite on Plan of Action as 2023 Farm Bill Deliberations Approach
FOR IMMEDIATE RELEASE
Key industry stakeholders convened at the NoCo9 Hemp Expo in March to finalize a policy document that 31 nonprofits have now signed.
WASHINGTON, D.C. (April 24, 2023) – As the 2023 Farm Bill deliberations approach, U.S. hemp leaders are united behind a plan of action. And this unprecedented alliance among 31 nonprofit hemp organizations portends promise for congressional enactment of the industry’s agenda.
This winter, three of the nation’s leading hemp organizations – Hemp Industries Association (HIA), National Industrial Hemp Council (NIHC) and U.S. Hemp Roundtable (USHR) – joined in collaboration for the very first time to develop a series of policy priorities for enactment in the 2023 Farm Bill. The three groups then asked industry leader Morris Beegle to convene a meeting of more than 75 key hemp stakeholders at the leading national hemp gathering that he produces, the NoCo Hemp Expo. After an intense discussion, and follow-up breakout groups to expound on the deliberations, a priority policy document was finalized. Since then, 31 state, regional and national nonprofit organizations have signed on in support.
The document, attached hereto, lists nine key policy priorities for consideration by Congress. These include requiring FDA to regulate hemp extracts such as CBD; easing the regulatory burden on hemp farmers; repealing the hemp felon ban from the 2018 Farm Bill; and addressing THC limits for hemp. This document is being shared with key members of Congress and will serve as the foundation for drafting legislative language to be included in the Farm Bill.
NIHC President and CEO Patrick Atagi praised the work of the hemp industry for broadly coming together to endorse hemp priorities and hemp-specific Farm Bill priorities. “’Working Together Works’ are true words taught to me by my mentor, former USDA Undersecretary William ‘Bill’ Hawks,” Atagi said. “I am glad to see the hemp industry come together; it is a sign of great things to come.”
“This is an historic moment for hemp,” stated Jonathan Miller, USHR’s General Counsel. “The five years since legalization have been challenging, and the 2023 Farm Bill is our next and best opportunity to take this industry a step forward. The unity within the industry is remarkable and telling: Our shared voice will resonate with Congress and help us turn this opportunity into meaningful progress for hemp farmers and product consumers.”
Said Morris Beegle, co-founder and president of We Are For Better Alternatives, or WAFBA: “The last five years have taught us a lot, and more than anything, that we as stakeholders need to align our interests and our voices going into the 2023 Farm Bill so that we correct the regulatory deficiencies that have plagued the growth and development of this nascent industry. I’m optimistic and encouraged by so many organizations coming together at this time to collaborate and work in unison to improve the future of the hemp industry.”
Contact
Morris Beegle, WAFBA, info@nocohempexpo.com
Steven Hoffman, Compass Natural, steve@compassnaturalmarketing.com, tel 303.807.1042
WAFBA Announces a Pause for 2023 Southern Hemp Expo in Nashville
SHE became dominated by Delta-8 and other intoxicating hemp-derived cannabinoids popular in areas where adult-use cannabis is not legal.
LOVELAND, Colo. (April 18, 2023) -- WAFBA -- We Are For Better Alternatives, the umbrella organization that hosts and produces NoCo Hemp Expo, Southern Hemp Expo, Hawaii Hemp Conference, Winter Hemp Summit and other hemp-centric conferences, trade shows and events -- is going to pause the 2023 Southern Hemp Expo. The Southern Hemp Expo, aka SHE, launched in 2018 in Nashville as the sister show to NoCo Hemp Expo, the most comprehensive hemp exposition and trade show on the planet.
SHE was a highly successful event in both 2018 and 2019 but was canceled in 2020 due to the Covid pandemic. SHE moved to Raleigh, North Carolina, in 2021 and back to Nashville in 2022. Both shows struggled to regain the momentum that the hemp industry had prior to the pandemic and both shows ended up being dominated by the rise in the intoxicating hemp-derived cannabinoid category, including Delta-8, HHC and other derivatives that have come to be popular with the consumer market in areas of the United States where adult-use cannabis is not yet legal.
Hemp for Health and Wellness
Both NoCo Hemp Expo and Southern Hemp Expo were founded on the decades-long mission and mantra that hemp is for health and wellness for humans and animals and an environmental benefactor for the planet in being able to produce eco-friendly, carbon-neutral to carbon-negative materials for a variety of industrial applications. These include commercial and residential construction products, textiles, automotive components, paper and packaging, bioplastics and biocomposites, animal bedding, biochar and more.
Another long-standing mantra for the hemp industry has been “hemp does not get you high,” which was the case until the domestic oversupply of CBD biomass increased significantly in 2019 and 2020. Additionally, the FDA continued to skirt its regulatory responsibilities given to the agency by Congress in the 2018 Farm Bill to regulate the burgeoning and popular CBD market. The lack of regulatory oversight kept major retailers and major consumer brands on the sidelines as well as investment money out of the industry. This in turn was a factor in the increased oversupply and the eventual development of the synthesized, hemp-derived cannabinoid market that is proliferating today. This market has opened up a variety of concerns, from consumer safety issues to state product bans as well as state legislation approving of these products, and other various regulatory murkiness around the country. To put it simply, it's a mess and extremely complicated to navigate in this new category of the hemp industry. To be clear, WAFBA, Southern Hemp Expo and its management are not in favor of "banning" or making "illegal" this new category of hemp-derived products. This category of products needs federal regulation, which we hope will occur in the upcoming 2023 Farm Bill.
Focus on Flagship Event
With the above-mentioned confusion, complexity and the state of intoxicating hemp-derived cannabinoids, WAFBA has made the decision to focus its trade show and event energy on its flagship event, NoCo Hemp Expo, and to do everything we can to build this event as the international hub and gathering spot of hemp-based heath, wellness, nutrition, environmental, and socially impactful opportunities that can benefit humanity and the planet.
For additional information and to keep abreast of future activities and events, we encourage the industry and interested parties to subscribe to our newsletter and visit our media platform at www.letstalkhemp.com.
Contact
Morris Beegle, WAFBA, info@nocohempexpo.com
Steven Hoffman, Compass Natural, steve@compassnaturalmarketing.com, tel 303.807.1042
Nutrition Industry Up in Arms Over Proposed Dietary Supplements Listing Bill; Could it Be a Slippery Slope to FDA “Preapproval?”
This article originally appeared in Presence Marketing’s June 2022 Industry Newsletter.
“We fully trust FDA will make mischief. We don’t know how, but we do have 60 years of history of FDA authority trying to limit access to supplements.” – Michael McGuffin, President, American Herbal Products Association, in response to S. 4090, the Dietary Supplement Listing Act of 2022
By Steven Hoffman
On April 26, 2022, U.S. Senate Majority Whip Dick Durbin (D-IL), introduced a bipartisan bill co-sponsored by Sen. Mike Braun (R-IN), S. 4090, the Dietary Supplement Listing Act of 2022.
The proposed legislation, according to a statement from Durbin’s office, would require dietary supplement manufacturers to list their products with the U.S. Food and Drug Administration (FDA), known as a mandatory product listing (MPL). In addition, companies would be required to “provide FDA with vital information about their products, including product names, a list of all ingredients, an electronic copy of the label, allergen statements, health and structure/function claims, and more. This information would then be made available to the public,” Durbin’s office said.
Introducing the bill, Durbin said, “70% of people in America take a dietary supplement, including me. I also believe that Americans who take vitamins, minerals, and herbs for their health and wellbeing have a right to know what’s in those supplements. Many people assume that if a product is sold in the United States of America, somebody has inspected it and it must be safe. Unfortunately, that’s not always true.”
Reaction to the bill among industry leaders and associations has been mixed, yet many in the nutrition industry are up in arms in that the bill proposes that all dietary supplements sold in the U.S. be listed in a federal database or registry. Opponents are concerned that it may infer that the FDA would end up gaining “pre-market approval” authority over dietary supplements, in that the bill states that upon receiving a company’s listing submission, FDA will “confirm” a “complete listing” and issue an identification number.
According to Nutritional Outlook, the questions dietary supplements companies are asking include: “What could cause FDA not to confirm a listing? Can products automatically go to market after submitting a listing, regardless of FDA’s response? Could FDA, in fact, use mandatory product listing as a premarket-approval lever to keep certain ingredients off the market?”
“This is purely premarket approval and anyone who tells you different is lying,” Daniel Fabricant , Ph.D., President and CEO of the Natural Products Association (NPA), told Nutritional Outlook. Based in Washington, D.C., NPA opposes the bill and has been galvanizing the dietary supplements industry to act quickly to send letters to voice their concerns to their legislators via a web page here.
“Senators Durbin and Braun are one step closer to creating pre-market approval for dietary supplements a reality. Their bill was recently tied to the FDA Safety and Landmark Advancement Act (FDASLA), creating new regulatory barriers and giving the FDA new authority to prevent health and wellness products from reaching millions of consumers. Sadly, Senators Braun and Durbin are using their dislike of the dietary supplement industry to misrepresent its excellent safety record. The FDASLA is intended for drugs, not supplements, and we cannot afford to allow critics to stifle the industry,” Fabricant said in an appeal to members.
NPA asserts that responsible natural products retailers and manufacturers already go to great lengths to ensure consumers have access to safe products. Operating under the framework of DSHEA, the Dietary Supplements Health and Education Act of 1994, “FDA has a robust regulatory framework to understand what dietary supplements are being sold and who is selling them,” NPA said.
“The FDA has several tools at its disposal, with associated penalties for failure to comply. Retailers and manufacturers also have strong market incentives to make safe products. The proposed language in the Dietary Supplement Listing Act of 2022 requires pre-market approval for dietary supplements, thus is more stringent than the NDI (New Dietary Ingredients) provision, which is a notification. The FDA already has access to information regarding who is making dietary supplements, where they are making them, what products are made at which facilities, when new ingredients are introduced into commerce, and whether any products are associated with serious adverse events,” the association added.
In a recent meeting of the U.S. Hemp Roundtable (USHR), representing CBD supplement companies, Michael McGuffin, President of the American Herbal Products Association (AHPA), said, “We oppose the bill because it is redundant, and we don’t think it is so perfectly written that it doesn’t drift into preapproval. I don’t see this as certain pre-market approval, but it says that FDA will confirm a submission that is complete, so what does ‘complete’ mean? FDA will interpret this as it sees fit. For example, you’re required to list all your claims, but they could say you didn’t list all your claims.”
One thing is certain, McGuffin said. “We fully trust FDA will make mischief. We don’t know how, but we do have 60 years of history of FDA authority trying to limit access to supplements,” he cautioned.
McGuffin added, “We like the word ‘listing’ vs. ‘registry,’ which sounds stronger, but Sen. Durbin said specifically ‘registration.’ Even though we all say listing because we agree not to whip people up over this, but the guy who wrote the bill, Sen. Durbin, said registration, Also, there is no exemption for retailers, so every white label brand also will have to register,” he noted.
“We understand there may be an inevitability here, and then we would switch our focus to how to make it least impactful,” McGuffin said, referring to the proposed legislation. “The details are going to be important, and there is a lot we don’t know in this bill.”
Megan Olsen, Senior VP and General Counsel for the Council for Responsible Nutrition (CRN), told USHR attendees that CRN supports the proposed Dietary Supplement Listing Act. Referring to a product registry, she said, “This could give consumers and retailers a place to look to see if products are legitimate, continuing to ensure that there is transparency over the industry and enhancing consumer trust. However,” she added,” our position is that it cannot be pre-approval. If it looks like it’s going that way, that’s when we would push back very strongly.”
In speaking of the proposed dietary supplements legislation, Karen Farrell, Senior Director of Brand Management, Nutrition and Body Care for Presence Marketing, said, “I feel like the industry should keep its antennas high on this one. We already have existing policy in DSHEA; if something needs to be changed, it should be done under the existing structure of DSHEA rather than write separate legislation outside of what already exists. We still have some people who say the supplement industry is unregulated, and that’s just not true,” she said, adding that the proposed bill could also slow product innovation and launch times.
“Dietary supplement manufacturers are already playing by the rules; they are jumping through all the regulatory hoops and have made a commitment to quality. I’ve been reviewing new products and brands for 10 years at Presence, and I think I only ever saw one brand that I had questions about, and we addressed it right away. FDA already has authority to regulate and send warning letters, etc. I don’t think we need this bill; DSHEA works, Farrell added.
# # #
Hemp Industry Shifts from CBD to Food and Fiber, According to New Report
Photo: Pexels
This article originally appeared in Presence Marketing’s February 2022 Industry Newsletter
By Steve Hoffman
Look for the industrial hemp market to continue to grow in 2022, in spite of a few handicaps caused by continued regulatory uncertainty from the FDA and supply chain bottlenecks, according to the new Hemp Industry 2022 Opportunities Report published by Let’s Talk Hemp Media. The executive-level report provides an overview of the trends and potential strategies ahead in the growing industrial hemp sector, after hemp has been legalized in more than 65 countries this year.
Two things holding the hemp industry back in the U.S. are supply-chain bottlenecks and regulatory uncertainty, said report contributor Beau Whitney of Portland, OR-based Whitney Economics. “Once the market begins to mature, the future looks bright for the hemp industry,” Whitney noted.
The pandemic and a glut in biomass supply saw farmers turn away from hemp last year. Licensed acreage in 2021 dropped by 55% year-over-year to levels seen before the 2018 Farm Bill, according to the report. CBD market prices fell below production costs, due to a glut of product, as well.
However, a “bright spot was the supply and demand for fibers and grain,” the report said. Expect a “pivot” in the hemp industry as more farmers see the potential (albeit less profitable) of hemp fibers and grains, which are predicted to exceed acreage planted for CBD by 2024-25, Whitney projected.
Regulatory uncertainty in the U.S. has hampered the cannabinoid hemp industry because the FDA to date has refused to approve CBD as an ingredient for food and beverage or dietary supplements. However, CBD remains popular with consumers, and recently passed legislation in California sets health guidelines for hemp-derived CBD in food, beverages and cosmetics. That could be the green light for large multinational food companies waiting for FDA approval to add CBD to consumer products, experts believe.
In addition, hemp seed is viewed today as a superfood containing complete protein and omega-3 and omega-6 essential fatty acids. Hemp protein is poised to become a staple ingredient in new plant-protein foods and is expected to grow to $109 billion by 2050, the report predicts.
The Hemp Industry 2022 Opportunities Report is available at LetsTalkHemp.com. The report is published by the producer of the 8th Annual NoCo Hemp Expo, the hemp industry’s most comprehensive trade show and conference, scheduled for March 23-25, 2022, in Denver, CO.